First COVID-19 Vaccine Fully Authorized By FDA
On Aug.23, 2021, the Food and Drug Administration authorized the Pfizer-BioNTech COVID-19 Vaccine for individuals 16 years of age and older.
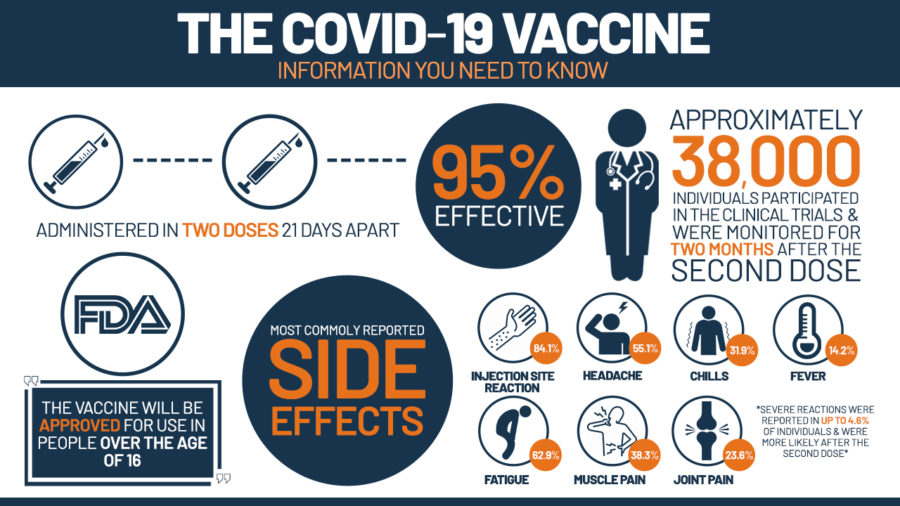
The FDA granted full authorization for the use of Pfizer BioNTech COVID-19 Vaccine, which is now being marketed as Comirnaty. This vaccine is administrated in the same way as Pfizer, two doses issued three weeks apart. Comirnaty is fully authorized for individuals sixteen years of age and older, while for individuals between the ages of twelve and fifteen the vaccine is still assigned emergency use authorization. Through the results of FDA studies and evaluations, the vaccine was shown to have a 91% effectiveness in preventing COVID-19 and associated conditions such as serious hospitalizations.
FDA Commissioner Janet Woodcock, M.D., said, “As the first FDA-approved COVID-19 vaccine, the public can be very confident that this vaccine meets the high standards for safety, effectiveness, and manufacturing quality the FDA requires of an approved product”.
In relation to Hawaii, due to the approval of Comirnaty, Lieutenant Governor Josh Green anticipates a new wave of residents desiring to receive the vaccination. Many companies in Hawaii –such as Hawaiian Electric, Hawaiian Airlines, and Bank of Hawaii –have instituted mandatory vaccinations for employees because of the FDA approval.
For more information on the testing, the FDA conducted to authorize this vaccine, how the vaccine works to prevent COVID-19, and the safety this vaccine provides, please visit:https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
To find where the vaccine is being administered in your community, please visit:https://www.vaccines.gov/search/

EMAIL: [email protected]
Hi! I am Sophie Bell, Grade 12. This is my first year on the Ka Mōʻī staff and I am super excited to get writing! I joined for the new experience this opportunity will bring to my high school experience, and I am planning to write interesting news stories and features.